How Do Plastic Or Paper Thermometers Register Body Temperature
Abstruse
Body temperature is a valuable parameter in determining the wellbeing of laboratory animals. All the same, using body temperature to refine humane endpoints during astute illness generally lacks comprehensiveness and exposes to inter-observer bias. Here we compared two methods to appraise body temperature in mice, namely implanted radio frequency identification (RFID) temperature transponders (method ane) to not-contact infrared thermometry (method 2) in 435 mice for up to 7 days during normothermia and lipopolysaccharide (LPS) endotoxin-induced hypothermia. There was excellent agreement between core and surface temperature every bit adamant past method ane and 2, respectively, whereas the intra- and inter-bailiwick variation was college for method 2. Nonetheless, using machine learning algorithms to determine temperature-based endpoints both methods had excellent accuracy in predicting death as an issue upshot. Therefore, less expensive and cumbersome non-contact infrared thermometry can serve as a reliable alternative for implantable transponder-based systems for hypothermic responses, although requiring standardization betwixt experimenters.
Introduction
The 3 Rs (Replacement, Reduction and Refinement) were introduced nearly 60 years ago as guiding principles for humane brute inquiryone. Since then, the application of humane end points, which permit early termination of experiments before animals feel meaning harm while intended to ensure robustness and reproducibility of results, has become widely accepted. In mice, the most unremarkably used laboratory animals, trunk temperature is a fundamental parameter in the evaluation of brute wellbeingtwo,3,4,five. It is therefore important in mouse models of astute illness to be able to reliably assess temperature. All the same, to date, but a minority of mouse studies using endotoxin or infectious agents to arm-twist acute illness apply temperature to monitor disease progression and define humane endpoints.
Traditionally, the mainstay of temperature monitoring in mice have been invasive measurement techniques such as rectal, tympanic or oesophageal probing and bladder or pulmonary artery catheterization allowing for measurement of core body temperaturesix,7. Of those, rectal thermometry is the most widely used means of temperature measurement in mice8,9. However, limitations of this procedure are its fourth dimension-consuming application and distress to animals, which may cause an increase in core temperature, leading to a misinterpretation of the animal's physiological statex,xi. In improver, rectal probing can lead to mucosal trigger-happy or infectionvii,8. In contrast, non-invasive temperature monitoring techniques such as non-contact infrared thermometry reduce brute discomfort and lower the run a risk for injury or cross contagion12. Common sites for surface temperature measurement in mice include the tympanic membrane, the back, sternum, abdominal region and ano-genital region, all of which correlate with core body temperature3,eight,13,14,15,xvi,17. More recently, subcutaneous implantable passive radio-frequency identification (RFID) or active telemetry transponder systems for core temperature probing have go a reliable alternative and are widely considered best practicesix,8,eleven. Nevertheless, in spite of its usability, the loftier startup expenses, required surgical skills and distress to animals acquired by the implantation procedure limit the widespread application of such systems.
The aim of this report therefore was to compare and assess the respective merits of two singled-out methods to assess torso temperature in mice during acute illness, namely the higher up implantable RFID transponder and non-contact infrared thermometry allowing for measurement of body core and surface temperature, respectively. To this end, nosotros used a well-established endotoxin (lipopolysaccharide, LPS) model, which induces acute sickness behaviour over a menses of around 36 hours accompanied by hypothermia. To let for meaningful comparison, a subgroup of the same animals was used in either method. Furthermore, to cover the entire severity spectrum of endotoxin-induced hypothermia, we used a big number of animals (due north = 435) and four different mouse strains, namely C57BL/six J and homozygous knockout strains for Mfge8, Mertk, and Cd11b (deficient for the phagocytic opsonin MFG-E8, the phagocytic receptor MerTK, and one subunit of the complement receptor 3, respectively), which demonstrate varying susceptibility to LPS. In addition, to appraise both methods during normothermia, we studied saline-treated controls and animals with long recovery afterward LPS application for up to seven days. To maximize generalizability of results, we used two different brands of standard non-contact infrared thermometers obtained at a local pharmacy to measure the animals' surface temperature. To compare the two methods, we performed correlation analyses between core and surface temperature and used a mixed furnishings model to evaluate the surface temperature equally a predictor of core temperature. Furthermore, nosotros trained auto learning models with the temperature data obtained by the 2 methods to determine advisable temperature thresholds and time points to be used equally humane endpoints in this model. We so compared the accurateness of both methods in the prediction of death as an outcome event.
Results
Temperature alter over fourth dimension in LPS-treated and saline-treated animals
Effigy ane shows the mean (95% CI) body core and surface temperature by measurement modality across both LPS-treated and saline-treated groups. As expected, LPS-treated animals showed a pronounced decrease in both surface and core temperature on both injection days. The surface temperature of LPS-treated animals measured with infrared thermometer 1 reached a minimum at 9 hours post-obit the kickoff injection (28.vi (ane.7) °C) and at 12 hours post-obit the second injection (29.0 (2.1) °C). Core temperature of these animals was the lowest at 12 hours following the commencement (33.9 (2.7) °C) and second injection (34.five (two.nine) °C), respectively. The surface temperature of LPS-treated animals measured with infrared thermometer 2 was the lowest at 10.5 hours following the first injection (27.2 (ane.8) °C) and 12 hours following the second injection (27.seven (two.4) °C). Cadre temperature of these animals reached the lowest value at 12 hours post-obit the first (33.half dozen (ii.4) °C) and second injection (34.3 (iii.ix) °C), respectively. Both core and surface temperature returned to baseline within 96 hours following the second LPS injection. Conversely, saline-treated animals showed a mild increase in both cadre and surface temperatures from baseline on both injection days, which tin exist attributed to treatment stress caused by the injection and repetitive temperature measurements. Cadre temperature of saline-treated animals reached its daily maximum at 4.v hours (38.0 (0.4) °C and 37.ix (0.v) °C) mail-injection on both injection days. Surface temperature of animals measured with infrared thermometer one peaked at six hours post-obit the first injection (32.4 (1.ii) °C) and at iii hours following the second injection (32.5 (0.8) °C), respectively. No significant increase in surface temperature from baseline was observed in saline-treated animals measured with infrared thermometer 2 (for details see Fig. one and Supplementary Tabular array S1).

Line graphs showing trunk cadre and surface temperature across lipopolysaccharide (LPS)- and saline-treated groups for up to 7 days post-handling. (a) Temperature profile obtained by implantable radio frequency identification (RFID) transponders and infrared thermometer 2 (n = 380; LPS-treated, 251; saline-treated, 129). (b) Temperature contour obtained past implantable RFID transponders and infrared thermometer 1 (due north = 55; LPS-treated, 33; saline-treated, 22.). Bluish, saline-treated command animals; red, LPS-treated animals; solid line, core temperature; dotted line, surface temperature; grey arrow, fourth dimension of LPS/saline injections. Data shown are means ± 95% CI.
Strain- or genotype- dependent effects on the core and surface temperatures were observed among LPS-treated animals (for details see Figure S1). Following the first injection, homozygous knockout and control animals showed a similar hypothermic response to LPS for core and surface temperatures alike. However, following the 2d injection, the hypothermic response to LPS was markedly less astringent in Cd11b knockout and control animals (35.half dozen (0.8) °C, 35.1 (2.5) °C, 31.7 (6.0) °C and 31.9 (6.0) for the lowest core temperature on injection mean solar day 2 for homozygous Cd11b knockout, pooled homozygous wildtype controls, Mertk knockout, and Mfge8 knockout animals, respectively). There was no significant genotype-dependent consequence on core temperature (p = 0.9, F = 0.18, repeated measures ANOVA). In contrast, we observed a genotype-dependent effect on surface temperature (p < 0.001, F = ten.ii). Interestingly, post-obit the second injection, Cd11b knockout animals had a significantly reduced surface temperature until the finish of the experiment when compared to controls (p < 0.001, repeated measures ANOVA). Thirty out of 284 LPS-treated animals (89%) survived for up to 7 days after the first injection (Fig. 2). Among the 30 dead animals, eighteen were found expressionless and 12 were euthanized after reaching predetermined humane endpoint criteria, illustrating a distinct weakness of the sickness behaviour score which was used for this study to identify an fauna'due south distress (Table 1, Supplementary Table S3). Death occurred from 24 to 192 hours after the first injection (average = lx.5 (35.i) h). Table i shows the mean (SD) last recorded temperature before death and fourth dimension of death per strain. All saline-treated animals survived.
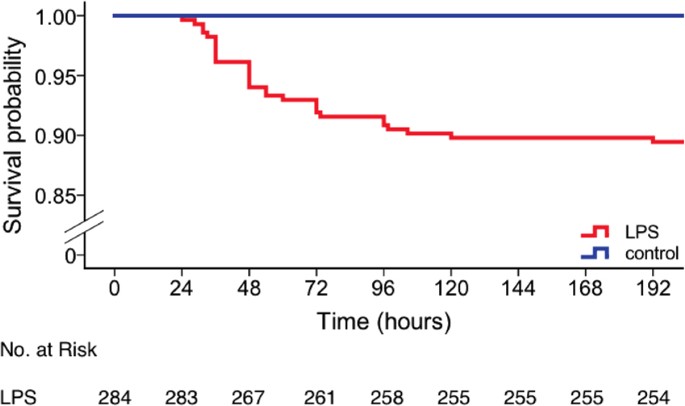
Kaplan-Meier bend showing cumulative survival beyond lipopolysaccharide (LPS)- and saline-treated animals for up to seven days post-injection. Bluish, saline-treated control mice (northward = 151, n(expressionless) = 0); crimson, LPS-treated mice (n = 284, n(dead) = thirty).
Comparison of measurement modalities
Surface temperature of the heating pad measured with infrared thermometer 1 was 1.vii °C higher than surface temperature measured with infrared thermometer 2 (infrared thermometer i: 37.5 (two.2) °C; infrared thermometer 2: 35.8 (0.9) °C), indicating unlike scale settings. Surface temperature across all animals was 5.7 °C (core temperature, 36.ix (1.9) °C; surface temperature, 31.2 (1.8) °C) and 6.9 °C (core temperature, 36.3 (2.v) °C; surface temperature, 29.4 (2.ane) °C) lower than cadre temperature for infrared thermometer one and 2, respectively (Fig. i).
Temperature acquisition past implantable RFID transponders was hampered by technical bug such as transponder readout error, dislodged and lost transponders, and reader failure leading to partial or total exclusion of 31, 14, and 6 out of a full of 199 transponder-implanted animals, respectively. In case of readout mistake or reader failure, bachelor information from before and/or afterward the technical problem was used in the analysis, which was divers every bit a fractional exclusion of experimental data. Animals with dislodged and lost transponders were entirely excluded from farther analysis. During surface temperature acquisition, an unexpected technical error by an untrained experimenter led to falsely increased readings in 13 Mfge8 mice on injection day 2. At 10.5 hours following the kickoff injection core and surface temperature values were non recorded in animals measured with infrared thermometer 1 because of human being error.
Correlation and prediction of core from surface temperature
At that place was loftier inter-method consistency between surface and core trunk temperature across all animals and treatment groups (intra-class correlation coefficient, ICC = 0.89; 95%CI: 0.88–0.ninety; n = 53 and 0.lxxx; 95%CI: 0.79–0.81; due north = 124 for infrared thermometer 1 and 2, respectively; Fig. 3).
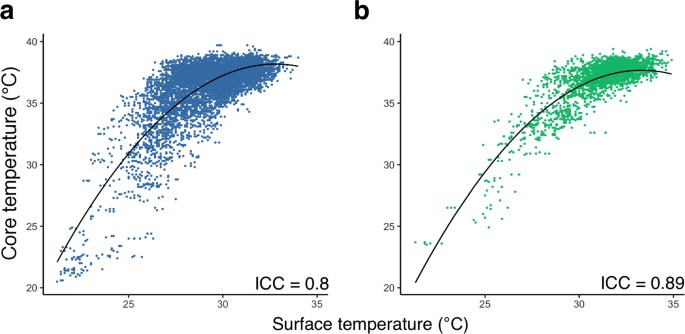
Prediction of core temperature from surface temperature using a mixed furnishings model. (a) Surface temperature measured by thermometer ii, plotted confronting the corresponding cadre temperature (northward = 124, ICC = 0.eighty (95%CI: 0.79–0.81)) and (b) surface temperature measured past thermometer model 1, plotted confronting the respective core temperature (n = 53, ICC = 0.89 (95%CI: 0.88–0.90)) bear witness a positive non-linear correlation. A fitted mixed effects model was used to predict the corresponding cadre temperature from surface temperature. Marginal R2 (R2m): 0.65, provisional R2 (R2c): 0.81; Black solid line, fit line of the mixed effects model showing the core temperature predicted from surface temperatur.\({\rm{Fit}}\,{\rm{line}}\,{\rm{in}}\,({\rm{a}}),y=-0.12\times {(x-30)}^{2}+0.92\times (x-30)+35.48\)\({\rm{fit}}\,{\rm{line}}\,{\rm{in}}\,({\rm{b}}),y=-0.12\times {(10-30)}^{2}+0.8\times (x-30)+36.36\)
A positive non-linear relationship was institute betwixt core and surface temperatures regardless of the infrared thermometer being used, indicating that the charge per unit of increment in core temperature slowed with higher surface temperatures. The nonlinear relationship betwixt surface and cadre temperature was potent at depression temperatures thus allowing for predictions of core temperature from surface temperature under hypothermic weather condition. However, the relationship was weaker at normothermia due to variation of the surface temperature.
Human relationship betwixt the cadre and surface temperature could be described as follows:
If core temperature is predicted from measures by infrared thermometer ane:
$$\begin{array}{rcl}core\_temperature & = & -0.12\times {(surface\_temperature-30)}^{ii}+\\ & & 0.8\times (surface\_temperature-30)+36.36\cease{array}$$
(1)
If core temperature is predicted from measures by infrared thermometer 2:
$$\begin{assortment}{rcl}core\_temperature & = & -0.12\times {(surface\_temperature-thirty)}^{2}\\ & & +0.92\times (surface\_temperature-30)+35.48\end{array}$$
(2)
In this study, the marginal Rtwo (R2m) was 0.65 and the conditional Rii (R2c) was 0.81, indicating a good model fit.
Using core temperature or surface temperature to predict death
To compare the accuracy of both core and surface temperature in the prediction of death as an issue event, we developed a temperature-based death prediction model using machine learning algorithms. To empathize whether models trained by cadre or surface temperature could achieve comparable performance in expiry prediction, a two-step approach was applied. First, core and surface temperatures from transponder-implanted animals were taken, and used separately to railroad train the prediction models (n = 160). Since surface temperature of just 3 dead mice was measured with infrared thermometer i, death prediction was not conducted separately for each infrared thermometer. Afterward a comprehensive parameter space search, comparing of cadre- and surface-temperature-based prediction models was conducted. This was followed by option of the best performing models, which were trained with data from all animals whose surface temperature measurements were bachelor at 36 hours after the first injection (n = 372; for details see Supplementary Table S2).
A parameter search with models including support vector motorcar, logistic regression and random forest classifier showed that an F1 score of >0.9 could only be achieved when temperature data of upwardly to 120 hours was available (Supplementary Table S2). With surface temperature data from 36 hours later on the first injection, the precision and F1 scores dropped by 0.06 and 0.08 when switching from a more complex support vector machine model to a decision tree model, respectively (northward = 160). However, the differences in precision and F1 scores dropped to 0 and 0.03 when data from additional animals was used (n = 372). The hateful prediction accuracy reduced by 0.01 when using a decision tree model instead of a support vector motorcar model for both sets of animals (due north = 160 and northward = 372, respectively; Supplementary Tabular array S2). Therefore, a decision tree model of depth one was used for its general performance and low complication. Animals that were not assessed 36 hours later the first injection were non included in the assay. All models were tested with multiple combinations of temperature values from dissimilar post-injection time points (for details see Supplementary Table S2).
Decease could be predicted with loftier accuracy both from core and surface temperature (accurateness = 96.three%, F1 = 0.77 and accuracy = 95.6%, F1 = 0.69 for core and surface temperatures, respectively; n = 160, number of dead animals = xiii). Surface temperature data from 372 mice (number of dead animals: 28) led to an accurateness of 96.5% and a F1 score of 0.76. Accordingly, there was fantabulous understanding betwixt core and surface temperature to predict death as an event result.
Using the above model, awarding of a temperature threshold of 28.1 °C or 24.3 °C (for core or surface temperature, respectively) at 36 hours afterward the showtime LPS-injection would have allowed for early termination of experiments for 13 out of 19 animals at this time point, thus avoiding otherwise unnecessary suffering and distress.
Discussion
The aim of this study was to compare and assess the respective merits of 2 usually used procedures to assess trunk temperature in mice during acute illness following an endotoxin challenge. 2 main strengths of this written report are the large number of animals used and that the two methods were evaluated within the aforementioned animals assuasive for an optimal comparison.
To summarize our results, both methods produced similar findings in mice during normothermia and following LPS-induced hypothermia and the inter-method consistency was high (Figs 1 and 3). Every bit expected and depicted in Fig. i, surface temperature was considerably lower than core temperature throughout the experiment. Surface temperature measured past infrared thermometer ane was higher than that of infrared thermometer 2 despite similar core temperature values indicating that the difference in surface temperature between the two infrared thermometers was most probable caused by different default scale settings. In addition, the variance of surface temperature measurements was virtually twice as high than that of cadre temperature (Supplementary Table S1). Withal, using a mixed model arroyo, core temperature could be predicted reliably from surface temperature and expiry as an consequence event could exist predicted with loftier accuracy and precision based on surface and core temperatures akin using auto learning algorithms (Figs 3 and 4). It would therefore announced from the nowadays study that although surface temperature measurements have higher degrees of variation and are less sensitive to subtle changes in temperature, this method is well suited to determine temperature-based humane endpoint criteria.
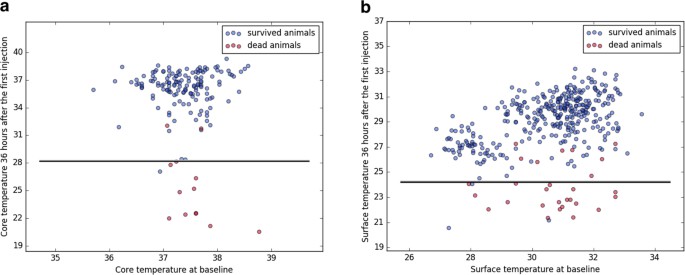
Predicting decease using threshold models trained with core or surface temperature. (a) Core temperature (n = 160, number of expressionless animals = thirteen) or (b) surface temperature (n = 372, number of dead animals = 28) at 36 hours after the outset injection was plotted against baseline temperature values. Blueish and ruddy dots correspond measurements of survived and dead animals, which were used in the training, testing and validation of the prediction model, with one dot indicating measurements of one brute. Blackness solid lines signal the conclusion boundaries determined past the prediction model trained with cadre or surface temperatures. If an animate being's body temperature falls into the surface area below the decision purlieus (i.e., core temperature <28.i °C or surface temperature <24.3 °C), the animal is predicted to die at a afterward fourth dimension point. Applying a combination of the two thresholds would take allowed for early on termination of experiments before animals experienced further distress for xiii out of 19 animals, which died at later time points during the study. Applying thresholds individually would have resulted in early termination of experiments for 4 out of 7 transponder-implanted animals or 12 out of 19 animals with surface temperature recordings, respectively.
Previous studies have addressed the correlation betwixt cadre temperature and surface temperature. Among studies involving core and surface temperature measurements in mice, the surface temperature is on average two.57 °C, 3–four °C or 2–3 °C lower than core temperature, depending on the site of measurement and restraints applied (sternum, dorsum without restraints and back with restraints, respectively) and there is moderate to potent correlation betwixt core and surface temperatures during hypothermia3,8,13. An important factor contributing to differences in absolute temperature betwixt an fauna's core and surface is ambient temperature. The lower the ambience temperature, the lower the surface temperature, whereas the core temperature stays constant as long equally thermoregulatory responses are intact18,19. Thus, variations betwixt studies can at least partly exist attributed to variations in ambient temperature. Furthermore, we and others have shown that surface temperature has poor predictive value for core temperature at normothermiavii,xx,21,22 (Fig. 3). This is most probable caused by changes in peel vasomotricity, which occur in phase with physiological thermoregulatory events, i.e. regular waves of vasoconstriction and vasodilatation of vessels in the skin in areas such as the paws and tail23. It is likely that the perineal area, which is close to the base of the tail and which was used for temperature measurement in this study, would be affected in the same way. In dissimilarity, surface temperature has good predictive value in hypothermia and hyperthermia when thermoregulatory responses are insufficient to maintain core temperature vi,xiii,17,23. Some other factor contributing to differences between core and surface temperature is handling stress. Restraining devices were used in previous studies for probe-based surface temperature acquisition, yet, body temperature may start to increase within seconds of a mouse existence restrained3,viii,ten,24. Stress results in activation of the sympathetic nervous arrangement, which in turn leads to increased thermogenesis and vasoconstriction of peel vessels resulting in divergence of cadre and surface temperature25,26,27. In the nowadays report, the perianal region was used for its accessibility and demand for only minimal beast handling during surface temperature conquering. Thus, the difference between core and surface temperatures tin exist attributed to variations in ambience temperature, measurement location and thermoregulatory responses, handling stress, and the intentional use of standard not-contact infrared thermometers without a calibration feature. All the same, we found a strong correlation between core and surface temperatures.
Genotype-dependent effects on torso temperature were near pronounced in homozygous Cd11b knockout animals whose hypothermic response to LPS was significantly reduced following the second injection, which might be attributable to a conditioning effect of repeated inflammatory stimuli. In add-on, Cd11b knockout animals had a lower surface temperature following the 2nd LPS injection which might be due to an altered thermoregulatory response of this genotype.
A threshold adamant past body temperature was used as a refined humane endpoint in previous studies. However, the threshold for determining the run a risk of death was selected based on a less exhaustive arroyo: for example, plotting the mortality confronting body temperature, using receiver operating feature (ROC) curves to evaluate the relationship between lowest recorded temperature versus survival, assay of sensitivity and specificity of decease prediction of sure cut-off values, or/and a pick based on average/everyman temperatures in treated versus untreated animals2,3,9,thirteen,15. These methods only allow a parameter search with big increments (0.5–1 °C) in the predictor (i.e., body temperature) in a less systematic manner, thus compromising the accurateness of prediction. To identify the parameter that could be used as the humane endpoint in the present study, we applied an automatized parameter search with finer increments in the predictor (0.01–0.1 °C) to approximate the threshold criteria. In addition, previous studies refrained from conducting a comprehensive assessment of prediction models (e.chiliad., random forests, decision tree, logistic regression and support vector machine) or parameters (due east.g., average surface/core temperature and/or lowest surface/cadre temperature at individual fourth dimension points or across several time points, respectively) and their various combinations for death prediction. To our noesis, this is the showtime study to determine a temperature-based threshold and to compare the prediction accuracies of core vs. surface temperature using machine learning algorithms in a mouse model of acute disease.
Factors other than variation of measurements need to exist considered when assessing the relative claim of these two methods. Regarding surface temperature measurements, a standard not-contact infrared thermometer and only minimal grooming and technical expertise are sufficient. In contrast, core temperature measurements using temperature transponders require a dedicated RFID organisation consisting of passive (usually non-reusable) RFID transponders and a reader device; transponder implantation needs to be performed past an experimenter with previous surgical experience followed by regular checks for transponder functionality and dislodgement. With respect to fourth dimension efficiency, non-contact infrared thermometry allows to obtain a measurement in iii–4 seconds, compared to ten–30 seconds for scanning and obtaining a measurement, including a re-calibration fourth dimension, using the RFID reader system as used here. Thus, in the easily of a skilled fauna technician, using infrared thermometry is likely to reduce handling time and animal distress. However, surface temperature measurements are prone to higher inter- and intra-field of study variation and highly investigator-dependent, whereas transponder-based readings are more robust and less investigator-dependent. Table ii summarizes the advantages and drawbacks of the two methods.
One important conclusion from our written report is therefore that, given the above constraints of using implantable RFID temperature transponders, infrared thermometry is adequate equally surrogate whenever variation of measurements tin can be balanced with multiple measurements or big numbers of animals. However, to quantify subtle changes in temperature requires the employ of the former despite being more than cumbersome, expensive, and time consuming. As the result, both core and surface temperature are equally suited to predict death assuasive for termination of experiments at before time points to reduce unnecessary distress.
Limitations of our study include the employ of merely one disease model, namely LPS-induced hypothermia. We chose an endotoxin model for its reproducibility and high translational relevance, as hypothermia is a mutual feature during astringent affliction in mice28,29,30. Information technology would still exist of interest to compare surface and cadre temperature measurements during hyperthermia every bit produced by stress or pharmacological intervention. Some other limitation is the proximity of the RFID transponder's implantation site to brown adipose tissue located in the interscapular region. Brown adipose tissue is an important oestrus generator in mice and thus might have a confounding effect on core temperature readings31. Just female mice were used for this study. However, the stage of the oestrous cycle was not determined. Thus, physiological temperature fluctuations with the oestrous bicycle may have had a confounding outcome on temperature measurements. Both core and surface temperature gave rise to comparable accuracy in decease prediction. However, due to the pocket-size number of animals that died in the nowadays study (6.9%), survived and dead cases were severely imbalanced, which may pb to an inflated accuracy estimate in death prediction. Therefore, nosotros calculated precision scores and F1 scores for models with high accuracy estimates to examine their performance in a more than comprehensive manner, which showed splendid agreement between the ii methods (Supplementary Table S2). Finally, considering of transponder malfunction and technical errors during temperature conquering, nosotros had to exclude core temperature measurements from 22 animals partially or in their entirety from farther analysis.
In conclusion, this is the first study to use systematic assessment of two distinct methods of temperature measurement in mice following an endotoxin challenge and to compare their predictive strengths towards death as an outcome consequence. Nosotros find that both methods are adequately suited for the prediction of decease, and hence that the less expensive and cumbersome not-contact infrared thermometry can serve as a reliable alternative for implantable transponder-based systems. This finding is of practical importance every bit it encourages adoption of elementary temperature measurement tools to monitor disease progression and apply humane endpoints in mouse models of astute illness.
Materials and Methods
All experimental protocols were approved by the Landesamt für Gesundheit und Soziales, Berlin (Reg 239/fifteen) and were conducted in accord with the German animal protection law and local fauna welfare guidelines. Reporting of the study complies with the Arrive (Animate being Enquiry: Reporting of In Vivo Experiments) guidelines32.
Animals, housing and husbandry
Female, two months quondam C57BL/6 J mice were derived from Charles River. Mertk (Jax: B6;129-Mertk tm1Grl/J), Cd11b (Jax: B6;129-Mertk tm1Grl/J, B6.129S4-Itgam tm1Myd/J, and Mfge8 33 (provided by C. Théry, INSERM 932, French republic) knockout mice were derived from The Jackson Laboratory and Hertie Institute for Clinical Brain Research, respectively, and bred locally. Female homozygous knockout mice and their homozygous wildtype littermates were used in experiments at the age of 8–10 weeks (total: n = 435. C57BL/6 J: n = 55; Mertk: n = 126; Cd11b: n = 126; Mfge8: due north = 128. Animals were kept in specific-pathogen-free (SPF) conditions according to FELASA regulations and grouping-housed with ad libitum access to food and water in type 3 polycarbonate cages equipped with environmental enrichment tools (blood-red transparent plastic nest box and brownish paper towels). During acute illness and recovery, animals were housed individually in custom-made polycarbonate cages (20 × 20 × 30 cm) from 48 h before the beginning injection until 72 h later on the second injection, after which they were returned to their home group cage. Room temperature was maintained at 23.0 ± 1.0 °C with a relative humidity betwixt 55 and 65%. Animals were kept under a 12:12 h light:dark bicycle (lights on: 20.00, lights off: 8.00) and were exposed to white noise at moderate intensity (65 dB) during the night phase (Dohm Sleepmate, Marpac Audio Machines, Wilmington, U.s.a.). To minimize confounding furnishings, injections and temperature measurements were scheduled at the same time each 24-hour interval and experimenters wore single-use coveralls (Microgard 1500, Ansell Microgard, Kingston Upon Hull, Britain), gloves and surgical masks whenever in contact with animals.
Methods to foreclose bias
Animals were randomized for handling, measurement modality, and survival times using the Research Randomizer tool (https://www.randomizer.org) by a researcher who was not involved in the injection procedure or temperature measurements. Information on strain and treatment group consignment was concealed from experimenters until the end of the study.
Exclusion criteria and humane endpoints
In that location were no specific exclusion criteria. A scoring system based on full general activity and response to stimuli was adapted from the murine sepsis score every bit shown in Supplementary Tabular array S3 to determine disease progression and humane endpoint criteria34. Severity of disease was scored on a scale from 0 to five (normal score, 0; maximum severity: five). When the general activity and response to stimuli of an animate being matched criteria from different severity levels, the average of the two severity levels was assigned as the sickness score. Upon reaching a score larger than 4 in one case or a score of 4 twice within 2 hours, animals were immediately removed from the muzzle and killed past cervical dislocation. On the two consecutive injection days, animals were scored eight times daily (8:00 to 20:00, every 90 min). On recovery days 2 and 3 after the first injection, iii times daily (8:00 to twenty:00, every half dozen h) and once a day (8:00) from postal service-injection day iii until the end of the experiment.
Transponder implantation, anaesthesia, and temperature measurement
For temperature acquisition using radio-frequency identification (RFID) technology, passive RFID transponders were implanted subcutaneously. When the passive RFID transponder is within read range, its internal antenna draws energy from the radio waves emitted past the reader. This energy powers the flake, which then sends data back to the reader. Before implantation, the glass-covered, biocompatible temperature transponders (dimension: 2 mm × 14 mm; model: IPTT-300 transponders; BioMedic Data Systems, Seaford, Us) were programmed with private identification numbers, loaded in a needle applicator device, and sterilized (Fig. 5a). Three weeks prior to injection, temperature transponders were implanted subcutaneously in the region between the scapulae as described previously13,35. Anaesthesia was induced with 2% isoflurane delivered in 100% oxygen for <45 south before the implantation procedure and injected once with meloxicam (1 mg/kg; Sigma-Aldrich, USA) for analgesia. Following implantation, mice were observed for upwards to 48 hours for signs of complications and temperature transponders were checked weekly for presence and functionality before the start of the experiment.
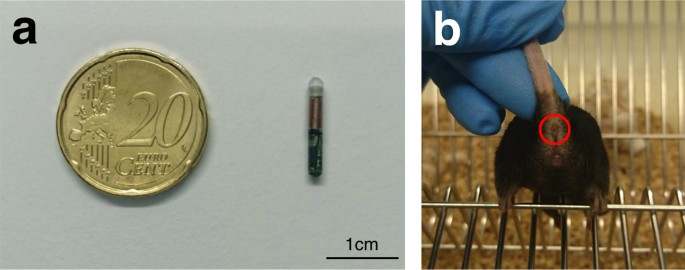
Illustration of the measurement modalities used to obtain core and surface temperature data. (a) Size comparison of a temperature transponder to a 20-cent money. (b) Mouse with the ano-genital expanse exposed. Red circle depicts perianal region used as the site for surface temperature acquisition.
A non-contact handheld transponder reader (DAS-7008/9; BioMedic Data Systems, Seaford, Usa) was used to read the implanted transponder. During temperature conquering, the fauna was placed on the experimenter's palm with the tail gently stock-still. The head of the handheld reader was held 2–3 cm to a higher place the animal's shoulder region, with a slow round motility until the temperature reading was displayed on the reader.
Non-contact infrared thermometry and temperature measurement
Non-contact infrared thermometers mensurate the infrared energy emitted by an object for estimating its temperature and tin only be used to monitor surface temperatures. In this report, two non-contact infrared thermometer models (model 1: Braun No touch – NTF3000; Braun, Kronberg, Germany; model 2: Aponorm Contact Gratis 3; WEPA Aponorm, Hillscheid, Germany) were obtained from a local pharmacy. For direct comparing, both thermometer models were initially used to mensurate the temperature of a heating pad (Hot Plate 062; Labotect, Göttingen, Germany) over a temperature range from 31 to 39 °C with an increment of 0.v °C. During subsequent temperature acquisition, the base of the tail was fixed with 2 fingers and and so gently lifted while the animal gripped a metal rod on the muzzle lid with its front paws, thus assuasive for exposure of the ano-genital area. Temperature was measured in the perianal region, with a searchlight indicating the measured area (Fig. 5b). To minimize misreckoning furnishings by urination or defecation, temperature measurements were just taken when the measurement area was clean. Infrared temperature measurements were taken over a readout time of approximately 3–iv seconds with the thermometer held i–2 cm from the reading site.
Timeline of temperature monitoring
Baseline body temperature readings were obtained at eight am at the 24-hour interval of the beginning injection. On the two consecutive injection days torso temperature was obtained eight times daily (8:00 to 20:00, every xc min), three times daily (viii:00 to 20:00, every vi h) on recovery days 2 and iii after the first injection, and once a twenty-four hour period (8:00) from post-injection day 3 until the end of the experiment (for details see Supplementary Figure S2). The sequence of core or surface temperature measurements was randomized. Treatment was minimized to reduce stress and discomfort.
Endotoxin-induced systemic inflammatory response
To induce a systemic inflammatory response, animals were treated with lipopolysaccharide (LPS), a cell wall component of Gram-negative bacteria. LPS (from Salmonella enterica serotype, Sigma-Aldrich St. Louis, United states of america) at a dose of 1.5 mg/kg or physiological phosphate-buffered saline solution were administered intraperitoneally on two consecutive days at the beginning of the agile (i.due east. light-off) phase at 8:00 with a volume of 10 μl/yard.
Experimental design
Experimental animals were divided into v groups with dissimilar survival times: iii hours (n = 44: 16 Mertk, 14 Cd11b and fourteen Mfge8), 1 day (n = 63: 10 C57BL/6, xix Mertk, 17 Cd11b and 17 Mfge8), 3 days (n = 76: x C57BL/vi, xviii Mertk, 25 Cd11b and 23 Mfge8), 7 days (n = 66: eleven C57BL/6, xvi Mertk, xix Cd11b and 20 Mfge8) and threescore days (n = 186: 24 C57BL/6, 57 Mertk, 51 Cd11b and 54 Mfge8), respectively. Survival times were adamant to fulfil the objectives of another study, for which these animals were used. Infrared thermometer 1 was used in the assessment of surface temperature in C57BL/half-dozen mice (northward = 55). Infrared thermometer 2 was used for temperature conquering in Mertk (n = 126), Cd11b (n = 126), and Mfge8 (n = 128) mice.
Data analysis and statistics
For all animals, temperature measurements were repeated in triplicate. Results are expressed every bit mean (SD) unless otherwise specified. Data processing and statistical analysis was performed using SPSS version 24 (SPSS Inc., Chicago, IL, USA), R 3.3.3 (R Development Core Team) and Python 2.7.ten (Python Software Foundation, https://world wide web.python.org). Intra-class correlation coefficients (ICC) and 95% confidence intervals (CI) were used to analyse the level of agreement betwixt surface and core temperature measures. To appraise the reliability of surface temperature in predicting the corresponding core temperature, a random intercept mixed effects model36,37 with three levels was used to fit the data (1st level, temperature measures; 2nd level, fourth dimension points where measures from unlike temperature monitoring methods were combined; third level, animals), for its advantage in dealing with missing values caused by unlike survival times and measurement intervals. Gamble of death every bit an result event was examined with the scikit-learn toolkit (sklearn)38 for both core and surface temperature. Cadre and surface temperature were analysed separately with (i) temperature data from 12 and 36 hours after the first injection; (two) lowest temperature by 24 hours for the first 48 hours post-injection (i.eastward., the lowest temperatures for hours 0–24 and for hours 24–48); and (three) average temperature by 24 hours for the offset 48 hours post-injection (i.e., the average temperatures for hours 0–24 and for hours 24–48). The three parameter sets were used individually or in combination with logistic regression model, decision tree model, back up vector machine, and random forest classifier with a 3-fold (due north = 160) or five-fold (north = 372) stratified cross-validation.
Data availability
3 datasets including (1) surface, core temperature and sickness score, (2) other data (treatment group assignment, strain/genotype and survival condition) of all animals and (3) temperature readings from a heating pad obtained with the two thermometer models are available equally open up information on Figshare Repository in raw data format: Figs 1, three and iv and Tabular array i (Dataset 1), https://doi.org/10.6084/m9.figshare.5589883; Fig. 2 and Tabular array 1 (Dataset 2), https://doi.org/10.6084/m9.figshare.5589892, temperature readings from a heating pad obtained with the two thermometer models (Dataset iii), https://doi.org/10.6084/m9.figshare.5765991.
References
-
Russell, W. G. Southward. & Burch, R. Fifty. The principles of humane experimental technique. (Methuen, 1959).
-
Kort, W. J., Hekking-Weijma, J. M., TenKate, 1000. T., Sorm, Five. & VanStrik, R. A microchip implant system as a method to decide torso temperature of terminally ill rats and mice. Lab. Anim. 32, 260–269 (1998).
-
Warn, P. A. et al. Infrared trunk temperature measurement of mice every bit an early predictor of death in experimental fungal infections. Lab. Anim. 37, 126–131 (2003).
-
Trammell, R. A. & Toth, Fifty. A. Markers for predicting death every bit an outcome for mice used in infectious disease inquiry. Comp. Med. 61, 492–498 (2011).
-
Hunter, J. Due east., Butterworth, J., Perkins, N. D., Bateson, M. & Richardson, C. A. Using body temperature, food and h2o consumption as biomarkers of disease progression in mice with Eμ-myc lymphoma. Br J Cancer 110, 928–934 (2014).
-
Goodwin, S. D. Comparison of Body Temperatures of Goats, Horses, and Sheep Measured With a Tympanic Infrared Thermometer, an Implantable Microchip Transponder, and a Rectal Thermometer. Journal of the American Clan for Laboratory Animal Scientific discipline 37, 51–55 (1998).
-
Brunell, M. K. Comparison of Noncontact Infrared Thermometry and iii Commercial Subcutaneous Temperature Transponding Microchips with Rectal Thermometry in Rhesus Macaques (Macaca mulatta). Journal of the American Association for Laboratory Animal Science 51, 479–484 (2012).
-
Newsom, D. M., Bolgos, G. L., Colby, L. & Nemzek, J. A. Comparison of trunk surface temperature measurement and conventional methods for measuring temperature in the mouse. Contemp Height Lab Anim Sci 43, 13–18 (2004).
-
Wong, J. P., Saravolac, E. K., Clement, J. K. & Nagata, 50. P. Evolution of a murine hypothermia model for study of respiratory tract flu virus infection. Lab. Anim. Sci. 47, 143–147 (1997).
-
Clement, J. Grand., Mills, P. & Brockway, B. Use of telemetry to record body temperature and activity in mice. J Pharmacol Methods 21, 129–140 (1989).
-
Quimby, J. G., Olea-Popelka, F. & Lappin, Grand. R. Comparison of digital rectal and microchip transponder thermometry in cats. J. Am. Assoc. Lab. Anim. Sci. 48, 402–404 (2009).
-
Shinozaki, T., Deane, R. & Perkins, F. M. Infrared tympanic thermometer: evaluation of a new clinical thermometer. Crit. Care Med. 16, 148–150 (1988).
-
Hankenson, F. C. et al. Weight Loss and Reduced Body Temperature Determine Humane Endpoints in a Mouse Model of Ocular Herpesvirus Infection. J Am Assoc Lab Anim Sci 52, 277–285 (2013).
-
Huang, H. P. & Shih, H. M. Utilise of infrared thermometry and upshot of otitis externa on external ear canal temperature in dogs. J. Am. Vet. Med. Assoc. 213, 76–79 (1998).
-
Gavin, H. Due east. & Satchell, K. J. F. Surface hypothermia predicts murine mortality in the intragastric Vibrio vulnificus infection model. BMC Microbiology 17, 136 (2017).
-
Bast, D. J. et al. Novel Murine Model of Pneumococcal Pneumonia: Employ of Temperature as a Measure of Illness Severity To Compare the Efficacies of Moxifloxacin and Levofloxacin. Antimicrob Agents Chemother 48, 3343–3348 (2004).
-
Dellavalle, B., Kirchhoff, J., Maretty, 50. & Castberg, F. C. & Kurtzhals, J. a. Fifty. Implementation of minimally invasive and objective humane endpoints in the study of murine <span class = "italic" >Plasmodium </span> infections. Parasitology 141, 1621–1627 (2014).
-
Kurz, A. Physiology of Thermoregulation. Best Practise & Inquiry Clinical Anaesthesiology 22, 627–644 (2008).
-
Sessler, D. I. Temperature Monitoring and Perioperative Thermoregulation. Anesthesiology 109, 318thesiologyio
-
Sikoski, P. et al. Comparison of rectal and infrared thermometry for obtaining body temperature in cynomolgus macaques (Macaca fascicularis). J. Med. Primatol. 36, 381–384 (2007).
-
Chen, P. H. & White, C. E. Comparison of rectal, microchip transponder, and infrared thermometry techniques for obtaining body temperature in the laboratory rabbit (Oryctolagus cuniculus). J. Am. Assoc. Lab. Anim. Sci. 45, 57–63 (2006).
-
Shelton, L. J., White, C. E. & Felt, S. A. A comparison of non-contact, subcutaneous, and rectal temperatures in captive owl monkeys (Aotus sp.). J. Med. Primatol. 35, 346–351 (2006).
-
El Bitar, Due north. et al. Thermoregulatory vasomotor tone of the rat tail and paws in thermoneutral conditions and its touch on a behavioral model of acute pain. J. Neurophysiol. 112, 2185–2198 (2014).
-
Krarup, A., Chattopadhyay, P., Bhattacharjee, A. K., Burge, J. R. & Ruble, Yard. R. Evaluation of surrogate markers of impending expiry in the galactosamine-sensitized murine model of bacterial endotoxemia. Lab. Anim. Sci. 49, 545–550 (1999).
-
Vianna, D. Thousand. 50. & Carrive, P. Changes in cutaneous and body temperature during and after conditioned fright to context in the rat. Eur. J. Neurosci. 21, 2505–2512 (2005).
-
Marks, A., Vianna, D. 1000. L. & Carrive, P. Nonshivering thermogenesis without interscapular dark-brown adipose tissue interest during conditioned fear in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R1239–1247 (2009).
-
Carrive, P., Churyukanov, One thousand. & Le Bars, D. A reassessment of stress-induced 'analgesia' in the rat using an unbiased method. Pain 152, 676–686 (2011).
-
Ochalski, S. J. et al. Inhibition of endotoxin-induced hypothermia and serum TNF-α levels in CD-ane mice by diverse pharmacological agents. Agents and Actions 39, C52–C54 (1993).
-
Vlach, M. D., Boles, J. Westward. & Stiles, B. G. Telemetric Evaluation of Body Temperature and Physical Activity as Predictors of Mortality in a Murine Model of Staphylococcal Enterotoxic Stupor. Comparative Medicine 50, 160–166 (2000).
-
Stiles, B. G., Campbell, Y. G., Castle, R. M. & Grove, S. A. Correlation of Temperature and Toxicity in Murine Studies of Staphylococcal Enterotoxins and Toxic Shock Syndrome Toxin 1. Infect. Immun. 67, 1521–1525 (1999).
-
Cannon, B. & Nedergaard, J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359 (2004).
-
Kilkenny, C., Browne, Due west. J., Cuthill, I. C., Emerson, Yard. & Altman, D. G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Fauna Inquiry. PLOS Biological science eight, e1000412 (2010).
-
Silvestre, J.-Southward. et al. Lactadherin promotes VEGF-dependent neovascularization. Nat. Med. eleven, 499–506 (2005).
-
Shrum, B. et al. A robust scoring system to evaluate sepsis severity in an animal model. BMC Res Notes vii, 233 (2014).
-
Caro, A. C., Hankenson, F. C. & Marx, J. O. Comparing of Thermoregulatory Devices Used during Anesthesia of C57BL/half dozen Mice and Correlations betwixt Torso Temperature and Physiologic Parameters. J Am Assoc Lab Anim Sci 52, 577–583 (2013).
-
Nakagawa, S. & Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods In Ecology And Evolution 4, (2013).
-
Johnson, P. C. D. Extension of Nakagawa & Schielzeth'south R2GLMM to random slopes models. Methods Ecol Evol five, 944–946 (2014).
-
Pedregosa, F. et al. Scikit-learn: Machine Learning in Python. Journal of Car Learning Inquiry 12, 2825–2830 (2011).
Acknowledgements
We thank all animal technicians from the Center for the Protection of Laboratory Animals at the High german Federal Found for Risk Assessment (BfR), peculiarly Paolo Rosellini Tognetti and Lisa Gordijenko, for their technical assistance. This study was funded past grants from Einstein Stiftung Berlin and Deutsche Forschungsgemeinschaft (DFG).
Writer information
Affiliations
Contributions
J.Five.E., S.B. and 1000.E. conceived, designed and supervised the report; J.M. and J.V.E. conducted the experiments; J.M. performed data acquisition, pre-processing and analysis under the supervision of N.R., U.Grand. and J.V.E.; J.Yard. and J.V.E. wrote the manuscript.
Corresponding author
Ideals declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's notation: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This commodity is licensed nether a Creative Commons Attribution iv.0 International License, which permits use, sharing, accommodation, distribution and reproduction in any medium or format, as long equally you give appropriate credit to the original author(due south) and the source, provide a link to the Creative Commons license, and point if changes were made. The images or other tertiary political party material in this article are included in the commodity's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you lot will need to obtain permission straight from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/iv.0/.
Reprints and Permissions
Nigh this article
Cite this article
Mei, J., Riedel, N., Grittner, U. et al. Body temperature measurement in mice during acute disease: implantable temperature transponder versus surface infrared thermometry. Sci Rep 8, 3526 (2018). https://doi.org/10.1038/s41598-018-22020-6
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/x.1038/s41598-018-22020-vi
Farther reading
Comments
Past submitting a annotate yous agree to abide by our Terms and Community Guidelines. If y'all discover something calumniating or that does non comply with our terms or guidelines please flag it equally inappropriate.
Source: https://www.nature.com/articles/s41598-018-22020-6
Posted by: greenabrount1980.blogspot.com


0 Response to "How Do Plastic Or Paper Thermometers Register Body Temperature"
Post a Comment